Department of Molecular Neuropharmacology

Scientific profile
- About department
- Employees
- Laboratories
Our research focuses on three areas: the reward system of the brain, the molecular mechanisms of drug action, and neuro-pharmacogenomics.
The reward system of the brain encompasses all brain areas involved in reward-driven behaviors, with the mesolimbic dopamine system at the core. We have been studying the mechanisms involved in the plasticity of dopaminergic and dopaminoceptive neurons, and also the role of endogenous opioids in signaling rewards.
As the name of the Department implies a large part of our research focuses on the cellular and the molecular mechanisms of actions of psychotropic drugs, also with an emphasis on opioids. We hope that elucidating molecular signatures of drug action will lead to the identification of mechanisms essential for their therapeutic effects.
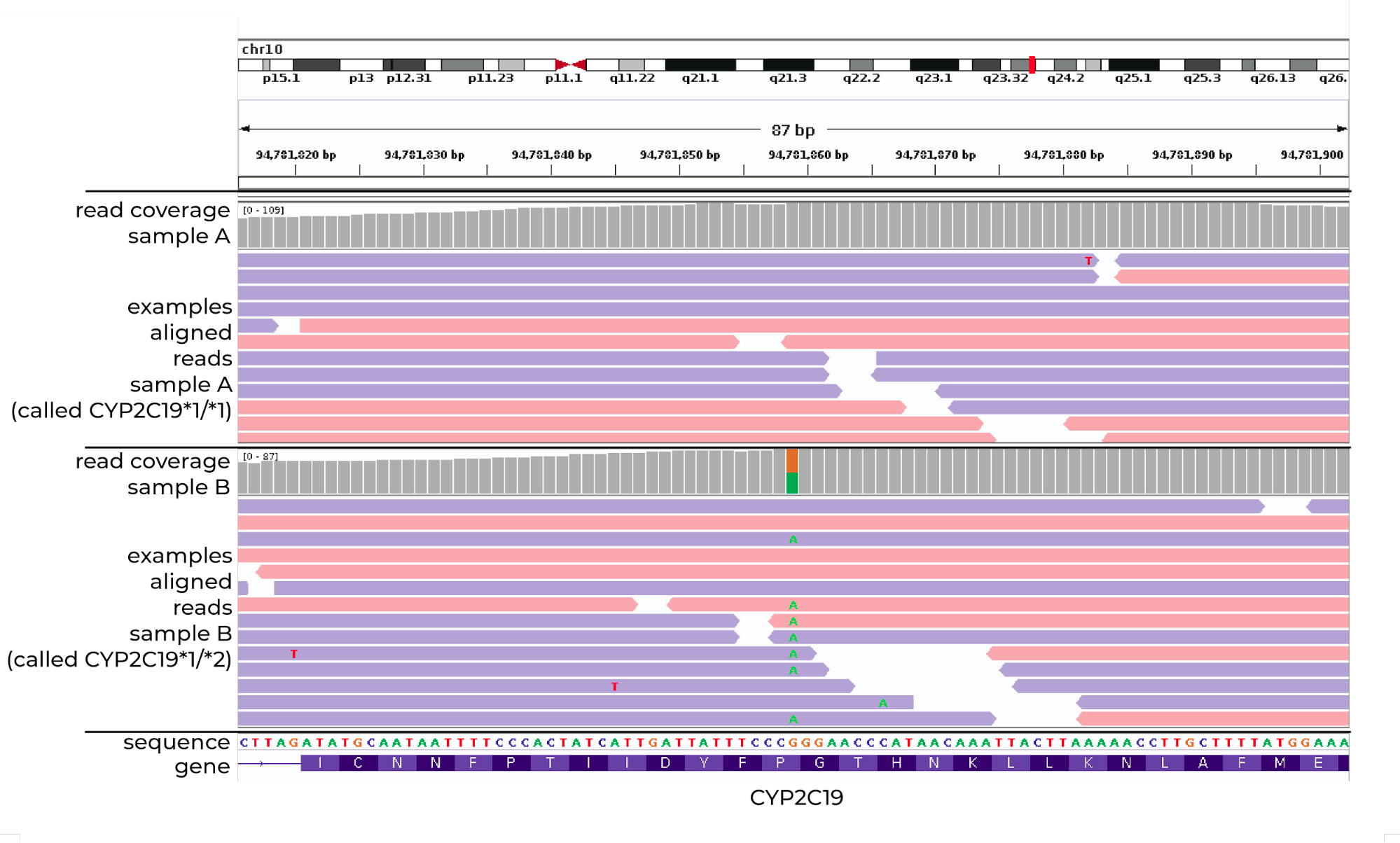
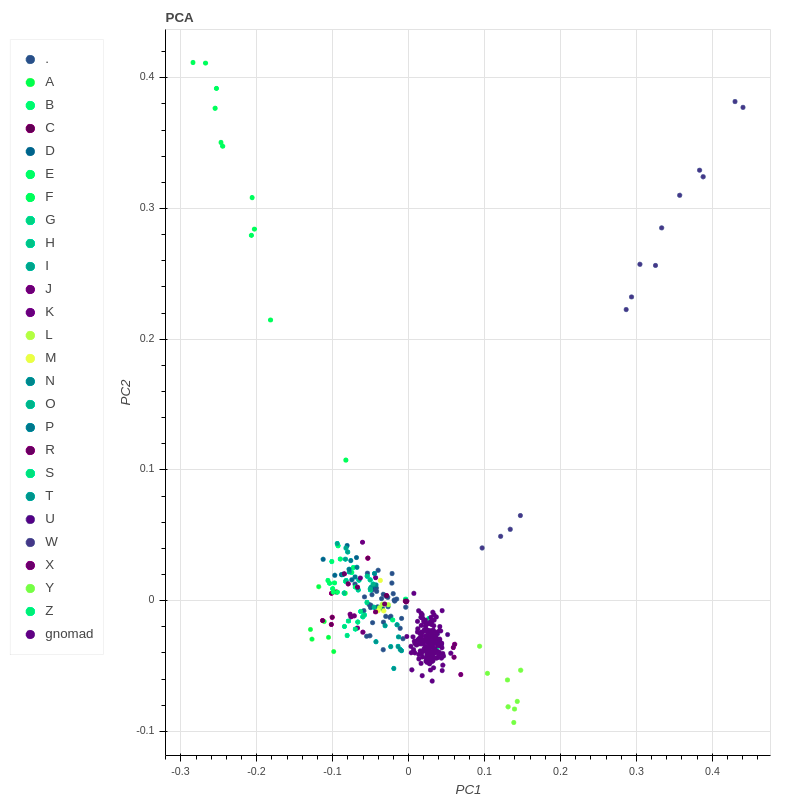
In recent years we have also been involved in neuro-pharmacogenomics, searching for associations between genetic background, neuropsychiatric disorders, and the effectiveness of pharmacotherapy. We use next-generation sequencing to discover novel common as well as rare genetic variants associated with drug effectiveness and safety. This is of particular interest in psychiatry due to large interindividual differences in therapy responses.


Professor Jan Manuel Rodriguez Parkitna, dr hab.
Head
Employees
Professor Ryszard Przewłocki, dr hab.
Michał Korostyński, dr hab.
Marcin Piechota, dr
Ganna Shayakhmetova, dr
Małgorzata Borczyk, dr
Sławomir Gołda, dr
Zofia Harda, dr
Alla Voronina, dr
Barbara Ziółkowska, dr hab.
Łukasz Szumiec, mgr
Lidia Radwan, mgr
Jacek Hajto, mgr
Klaudia Misiołek, mgr
Magdalena Ziemiańska, mgr
Mateusz Zięba, mgr
Employees
Michał Korostyński, dr hab.
Marcin Piechota, dr
Małgorzata Borczyk, dr
Jacek Hajto, mgr
Mateusz Zięba, mgr
Achievements
- Publications
- Grants
- Awards
Grant
The developmental changes in the endogenous opioid system associated with altered sensitivity to reward during adolescence
Klaudia Misiołek, MSc
Grant
Kappa opioid receptors integrate neuronal signaling involved in social behavior
Professor Jan Manuel Rodriguez Parkitna, PhD
Award
The Jerzy Konorski Team Award for the best study in neurobiology conducted in Poland awarded every year by the Polish Neuroscience Society and Committee of Neurobiology of the Polish Academy of Sciences
Professor Jan Manuel Rodriguez Parkitna, PhD
CREB activity in dopamine D1 receptor expressing neurons regulates cocaine-induced behavioral effects.
Bilbao A, Rieker C, Cannella N, Parlato R, Golda S, Piechota M, Korostynski M, Engblom D, Przewlocki R, Schütz G, Spanagel R, Parkitna JR
DOI: 10.3389/fnbeh.2014.00212
Neuroprotective effects of metabotropic glutamate receptor group II and III activators against MPP(+)-induced cell death in human neuroblastoma SH-SY5Y cells: the impact of cell differentiation state.
Jantas D, Greda A, Golda S, Korostynski M, Grygier B, Roman A, Pilc A, Lason W
DOI: 10.1016/j.neuropharm.2014.03.019
Novel drug-regulated transcriptional networks in brain reveal pharmacological properties of psychotropic drugs.
Korostynski M, Piechota M, Dzbek J, Mlynarski W, Szklarczyk K, Ziolkowska B, Przewlocki R
DOI: 10.1186/1471-2164-14-606
Controlling complexity: the clinical relevance of mouse complex genetics.
Schughart K, Libert C, SYSGENET consortium, Kas MJ
DOI: 10.1038/ejhg.2013.79
Full inhibition of spinal FAAH leads to TRPV1-mediated analgesic effects in neuropathic rats and possible lipoxygenase-mediated remodeling of anandamide metabolism.
Starowicz K, Makuch W, Korostynski M, Malek N, Slezak M, Zychowska M, Petrosino S, De Petrocellis L, Cristino L, Przewlocka B, Di Marzo V
DOI: 10.1371/journal.pone.0060040
Gene expression profiling of blood in ruptured intracranial aneurysms: in search of biomarkers.
Pera J, Korostynski M, Golda S, Piechota M, Dzbek J, Krzyszkowski T, Dziedzic T, Moskala M, Przewlocki R, Szczudlik A, Slowik A
DOI: 10.1038/jcbfm.2013.37
Astrocytes are a neural target of morphine action via glucocorticoid receptor-dependent signaling.
Slezak M, Korostynski M, Gieryk A, Golda S, Dzbek J, Piechota M, Wlazlo E, Bilecki W, Przewlocki R
DOI: 10.1002/glia.22460
Association between the A107V substitution in the δ-opioid receptors and ethanol drinking in mice selected for high and low analgesia.
Sacharczuk M, Lesniak A, Lipkowski AW, Korostynski M, Przewlocki R, Sadowski B
DOI: 10.1111/adb.12030
Training-induced acceleration of O(2) uptake on-kinetics precedes muscle mitochondrial biogenesis in humans.
Zoladz JA, Grassi B, Majerczak J, Szkutnik Z, Korostyński M, Karasiński J, Kilarski W, Korzeniewski B
DOI: 10.1113/expphysiol.2012.069443
Genotype-dependent consequences of traumatic stress in four inbred mouse strains.
Szklarczyk K, Korostynski M, Golda S, Solecki W, Przewlocki R
DOI: 10.1111/j.1601-183x.2012.00850.x